IBBC UMP Application Procedures Guide
INTRODUCTION OF IBBC
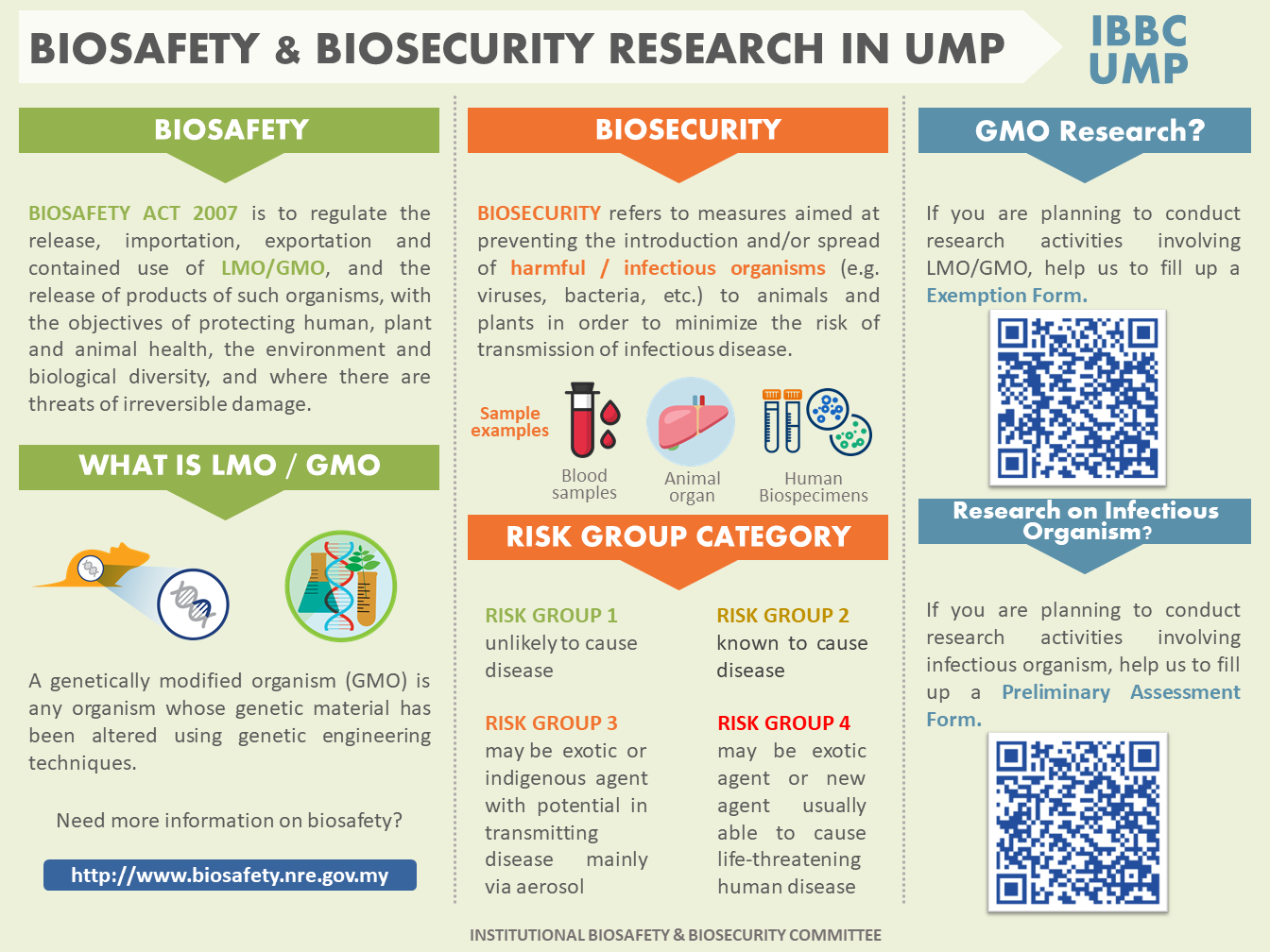
The field of microbiology has contributed significantly towards the development of modern medicine and biotechnology. Microbes including viruses, bacteria, fungi and parasites have been studied and utilized in the laboratory to help in our understanding of life and disease processes. While in most instances, innocuous or harmless microbes are used or encountered, there are instances in which there is a need to handle and identify potentially unknown infectious agents/materials and microbial toxins, or known virulent pathogens for research and the development of therapeutics, vaccines and preventive measures against the pathogens. Under these circumstances, it is of paramount importance that the environment in which the microbes or potentially unknown infectious agents/materials and microbial toxins are handled ensures safety to the personnel, public at large, protects the environment and in compliance to all the national and international obligations and legal frameworks which govern such activities. There is also a need to ensure that the microbes and potentially unknown infectious agents and microbial toxins are properly managed and safeguarded to prevent intentional and unintentional release that could cause harm.
IBBC Application Procedure
The applicant must obtain approval from the IBBC before the initiation of any activities that involve the handling, manipulating, working, using, storing, transporting and disposing of infectious and potentially infectious agents/materials and biological toxins. All applicants advised to firstly read and understand UMP policy and procedure prior to applying.
INTRODUCTION OF IBC
The IBC of University Malaysia Pahang has been initiated in view of the rapid development of projects involving modern biotechnology activities. It is foremost the ethical responsibility of the university to ensure research involving materials defined as biologically hazardous to be treated with extreme care to be safe for the primary user as well as the general population as a whole. Next, IBC has a major role to abide to the law governing biosafety i.e. Biosafety Act 2007. An Act to establish the National Biosafety Board; to regulate the release, importation, exportation and contained use of living modified organisms, and the release of products of such organisms, with the objectives of protecting human, plant and animal health, the environment and biological diversity, and where there are threats of irreversible damage, lack of full scientific evidence may not be used as a reason not to take action to prevent such damage; and to provide for matters connected therewith. The IBC takes pride in assisting National Biosafety Board (NBB) of Malaysia in upholding integrity of this Act. To be able to do so, IBC provides below the procedures and the necessary documentations for the approval to import, export, contained or non-contained use of such mentioned materials.
IBC Application Procedure
In the general the applicant being the responsible person require to provide vital input throughout the procedures. The integrity of the information provided will ensure a rapid processing towards a successful application. The procedures may be tedious but necessary due to the nature of the materials involved.